Ich Gcp Electronic Data Capture
In clinical trials the traditional paper-based data collection is replaced more and more by Electronic Data Capture EDC. As the clinical research world becomes increasingly technologically advanced we have seen more and more sponsors choose to use Electronic Data Capture EDC Systems to document Case Report Form CRF data.
See ICH GCP Section 8 for.
Ich gcp electronic data capture. ICH GCP Introduction 184This guideline has been amended to encourage implementation of improved and more efficient approaches to clinical trial design conduct oversight recording and reporting while continuing to ensure human subject protection and data integrity. 9 ELECTRONIC RECORDS. FEATURE Electronic Data Capture In VICH GCP Animal Health Studies DONNA WATSON.
The sponsor should base their approach to validation. According to ICH GCP 649. OCT 13 Quasar 11 FEATURE Of course EDC confers many other.
- For cause audit Een audit om een. The sponsors operating such computer systems must validate their systems maintain SOPs for their use ensure an audit trail for each data change and provide for data security The International Council for Harmonisation 1996. This is due to the belief in a faster turnaround of the Final Study Report after data collection has been made.
Electronic Data Capture EDC is becoming the data collection method of choice in Animal Health studies conducted to VICH GCP1. Interrogation of the data and its associated metadata will become much more commonplace on inspection however it is important to remember that the GCP principles we inspect against remain the same whether we are looking at paper or electronic source data. Excellent organizational and interpersonal skills.
Data from clinical. What can EDC provide in clinical t. Requirements for source data.
In depth knowledge of current ICHGCP guidelines applicable to the conduct of clinical research. The following issues have been observed by GCP inspectors regarding certain standards to be adhered to by the vendor. It is unclearnot mentioned.
The clinical trial applications are frequently incomplete regarding information on contracting out electronic data capture andor randomisation. We have found on inspection that user. Section 2 - The Principles of ICH GCP Reflecting modernization from paper-based documentation to electronic systems section 210 includes a minor clarification to indicate that clinical trial information irrespectiveof the type of media used should be recorded handled and stored in a way that allows its accurate reporting interpretation and verification.
Quality of the data produced. ICH GCP section 5 describes some requirements for the use of electronic data capture EDC eg. EDC-systeem Electronic Data Capture systeem Het systeem waarin data vastgelegd wordt dat ingevoerd is via eCRFs of elektronische vragenlijsten.
An eSource system can be considered as an EDC Electronic Data Capture system. This is also critical when organisations are designing new electroniccomputerised systems. Excellent attention to detail.
3 Figure 1 but research also suggests that while conducting 100 SDV of this data less than three percent of all case report forms CRF data is actually changed due to post-data capture monitoring and data. Good Clinical Practice - A Guide to Archiving Page 3 identification of the minimum list of essential documents and the responsibilities for their retention. Requirements and ICH GCP validated tested for user acceptability secure and maintained.
April 3 2019. FDA published a guidance in September 2013 to address the questions associated. Electronic Data Capture - Principal Investigator Signatures.
Group of suitably qualified and experienced people who review and evaluate the science medical aspects and ethics of a proposed. The guideline was developed with consideration of the current good clinical practices of the European Union Japan and the United States as well as those of. Standards to be followed.
The objective of this ICH GCP Guideline is to provide a unified standard for the European Union EU Japan and the United States to facilitate the mutual acceptance of clinical data by the regulatory authorities in these jurisdictions. 553 When using electronic trial data handling andor remote electronic trial data systems the sponsor should. In depth knowledge of issuesconsiderations involved with collecting safety data in an electronic data capture environment.
Electronic data capture EDC systems remain the primary source of data to be reviewed. The emphasis on data. - Essentiële documenten Documenten die het elk afzonderlijk en als geheel mogelijk maken de uitvoering van een klinisch onderzoek en de kwaliteit van de verkregen gegevens te evalueren zie hfd.
Health Canada is pleased to announce the implementation of International Council for Harmonisation of Technical Requirements of Pharmaceuticals for Human Use ICH Guidance E6R2. EDC is the current technology used by research institutions sponsors and CROs to manage clinical trial data when using electronic trial data handling andor remote electronic trial data systems. Page 3 Outline Computerized systems Electronic Records Acceptance of Electronic Data 21 CFR Part 11 Regulatory Expectations Expectation in GCP Inspections Data Audits.
Standards regarding electronic records and essential documents intended to increase clinical trial quality and. This guidance has been developed by the appropriate ICH Expert Working Group and has been subject to consultation by the regulatory. A Ensure and document that the electronic data processing systems conforms to the sponsors established requirements for completeness accuracy reliability and consistent intended performance ie.
Ability to reason independently and effectively problem solve complex issues.

4 Ways To Improve Clinical Data Quality In The Digital Era

Electronic Data Capture In Clinical Trials Pdf Free Download
Https Www Leukemia Net Org Network Services Informatics Services Electronic Data Capture E4663 Infoboxcontent4664 Macro 2007 01 29 Pdf
Https Www Ema Europa Eu En Documents Regulatory Procedural Guideline Draft Guideline Computerised Systems Electronic Data Clinical Trials En Pdf

4 Ways To Improve Clinical Data Quality In The Digital Era

Ich Gcp E6 Addendum R2 What Do You Need To Know

Ich Gcp E6 Addendum R2 What Do You Need To Know

Ecrf Electronic Data Capture Datacapt
Pestronk Electronic Data Capture Selecting An Edc System Journal Of The Society For Clinical Data Management
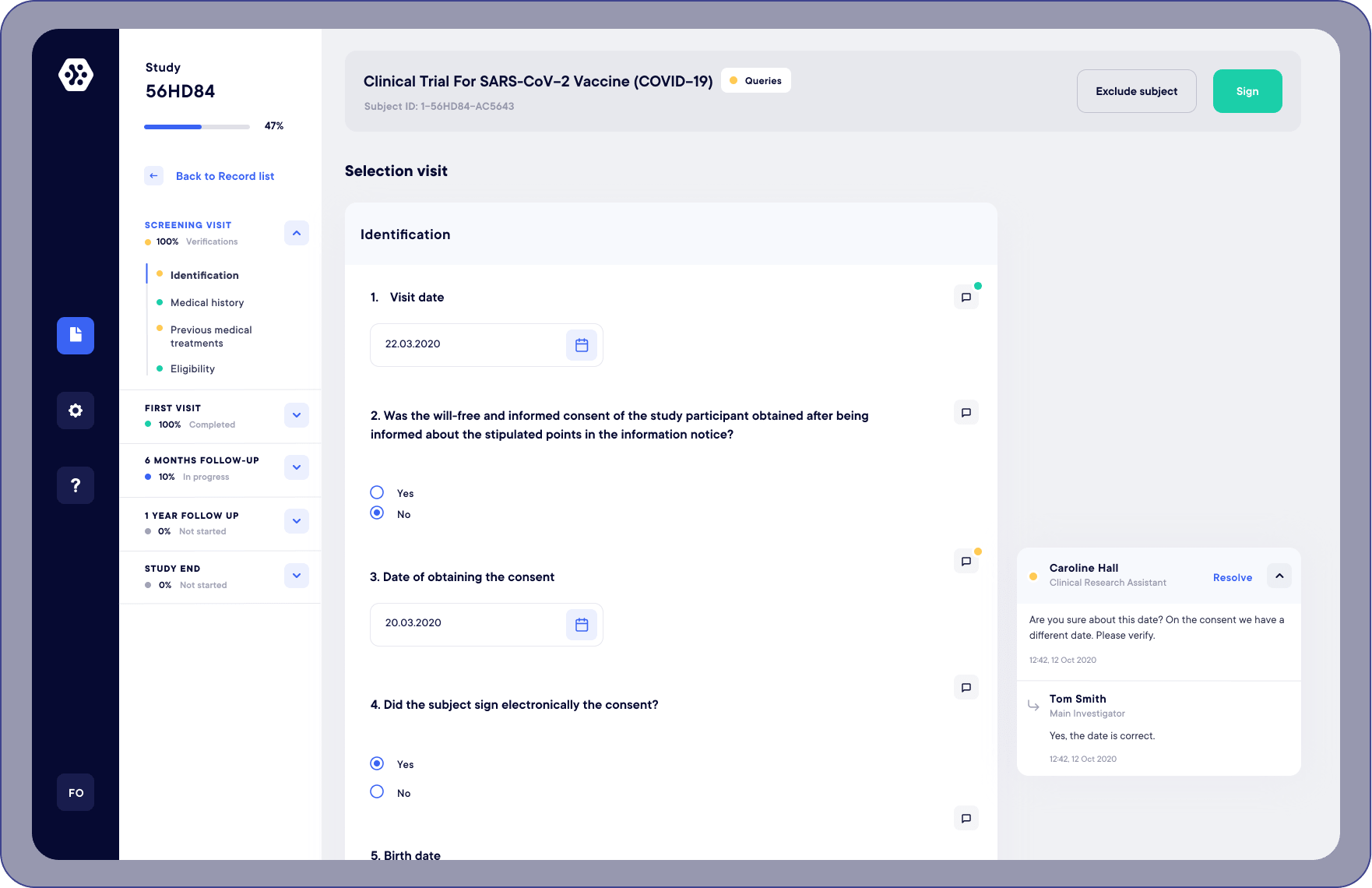
Ecrf Electronic Data Capture Datacapt
Https Www Ema Europa Eu En Documents Regulatory Procedural Guideline Qualification Opinion Esource Direct Data Capture Ddc En Pdf

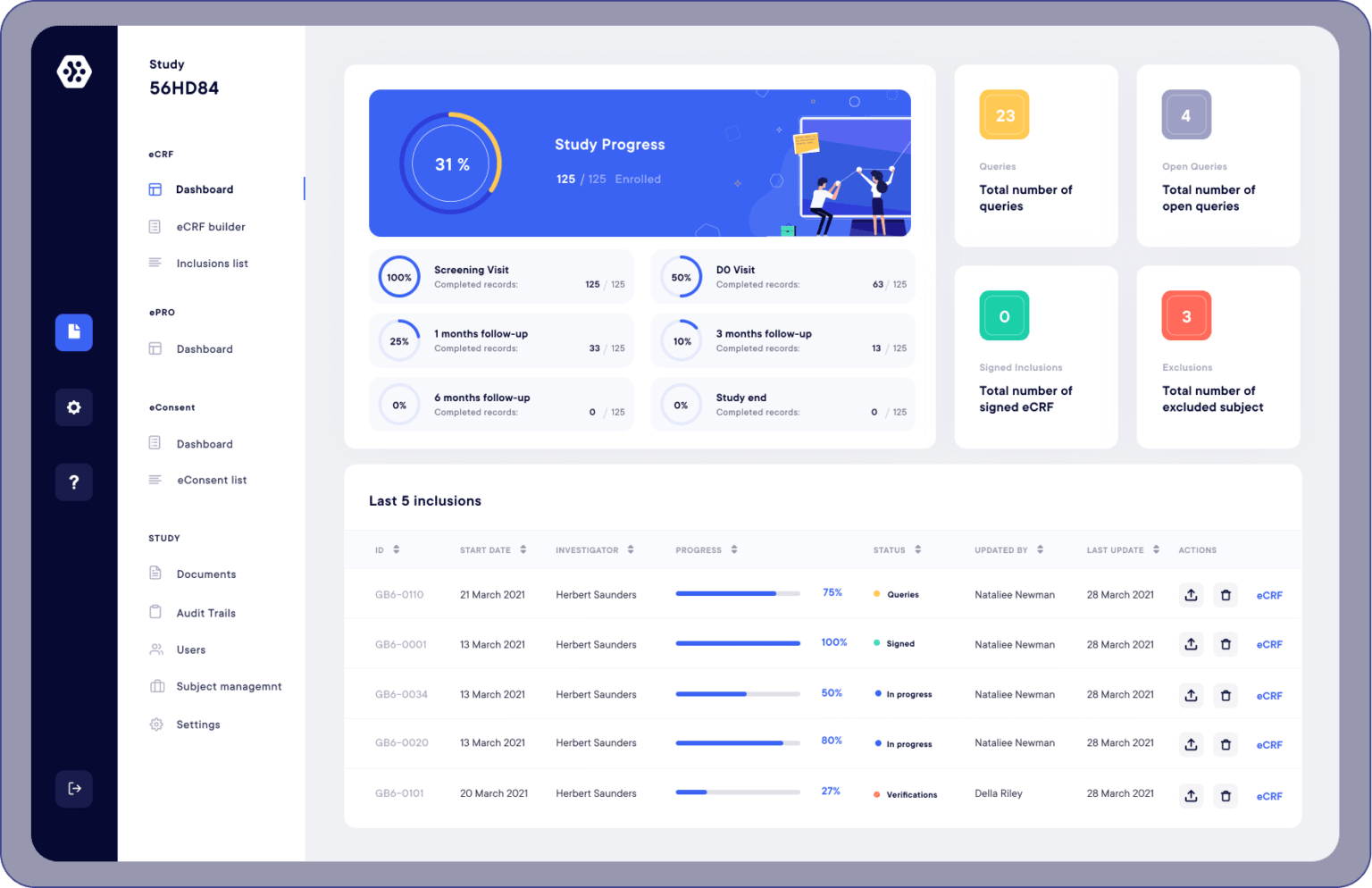
Ecrf Electronic Data Capture Datacapt
Datacapt The Best Clinical Research Platform Edc Econsent Ecrf Epro
Https Www Ema Europa Eu Documents Regulatory Procedural Guideline Draft Qualification Opinion Esource Direct Data Capture Ddc En Pdf

Clinical Trials Digital Technology For Recruitment Consent And Data Capture Focus On Regulation
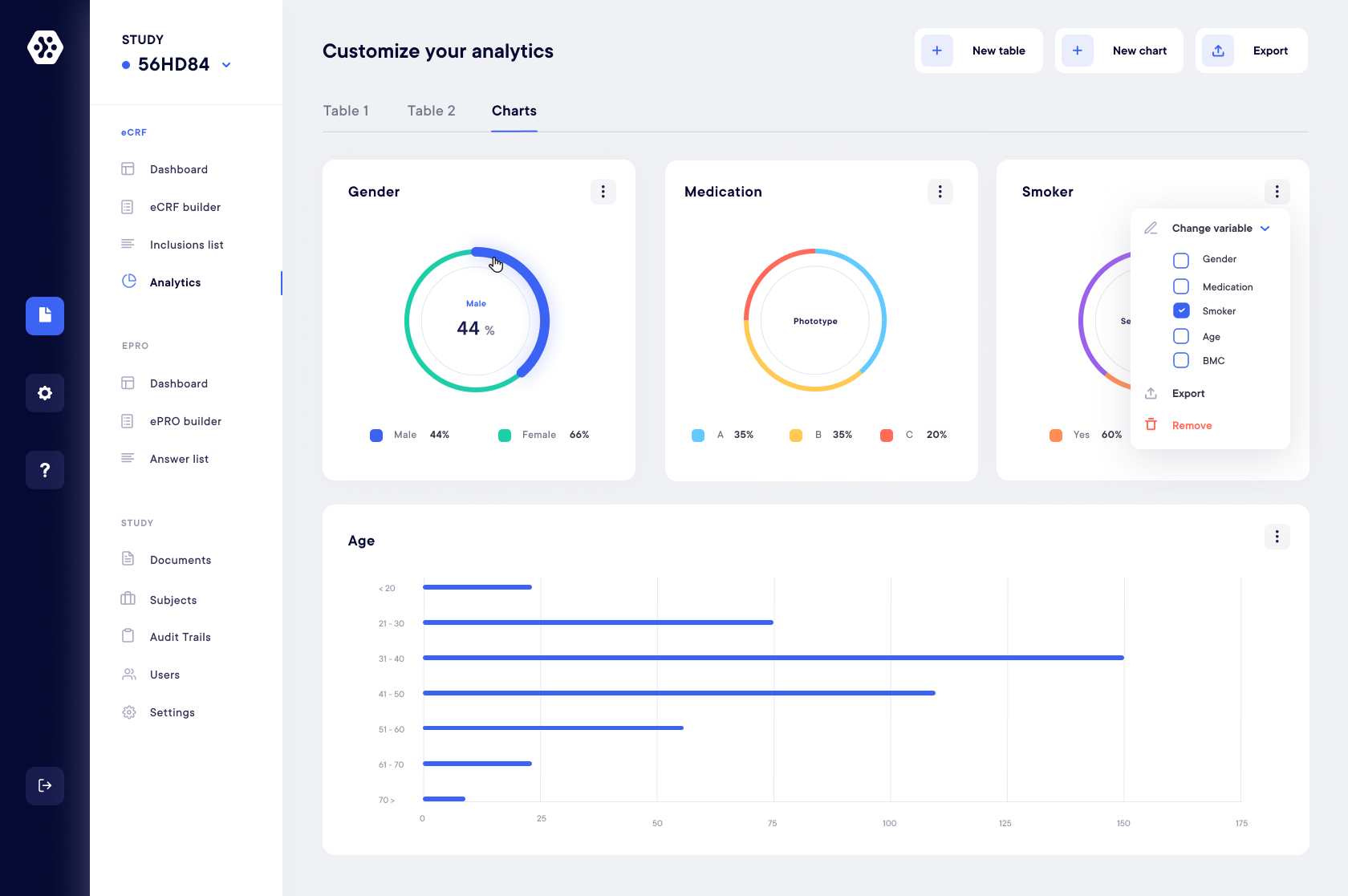
Ecrf Electronic Data Capture Datacapt
Etmf Electronic Trial Master File Software Ennov Software For Life
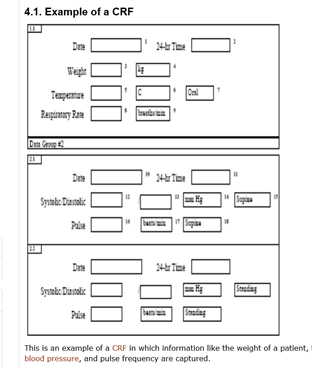

Post a Comment for "Ich Gcp Electronic Data Capture"