How Does Electronic Data Capture Work
What is Electronic Data Capture. At OpenClinica we are immersed in EDC all day every day.
Https Www Ema Europa Eu En Documents Regulatory Procedural Guideline Draft Guideline Computerised Systems Electronic Data Clinical Trials En Pdf
In addition to data capture we support custom age gates to allow you to prompt guests to ensure they are a certain age before using the app.
How does electronic data capture work. Electronic records like those created by electronic data capture systems are more easily searched and analyzed. More and more clinical trials are making the move to EDC software and replacing paper records with electronic records. Provides you with the ability to track manage and monitor disclosures on an ongoing basis.
To put it simply an Electronic Data Capture EDC system is software that stores patient data collected in clinical trials. An EDC system uses technology to streamline the collection and transmission of clinical trial data from the patient to the research laboratory. Annonce STRUCINSPECT DCE Data Capture Expert training for remote data capturing.
Theyll automatically receive a text or email with a link to their photo by filling out your data capture form. Electronic data capture EDC software digitalizes the data capture process for field teams surveyors researchers and study coordinators so they can do away with manual ways of collecting data. Annonce Corporate conflict management software.
However its important to recognize that many participants in the field of clinical trials are still just. More generally data capturing can also refer to collecting relevant information whether sourced from paper or electronic documents. Data capture can be set to required by disabling all send options.
Annonce Corporate conflict management software. Address common challenges with best-practice templates step-by-step work plans and maturity diagnostics for any Electronic data capture related project. Address common challenges with best-practice templates step-by-step work plans and maturity diagnostics for any Electronic data capture.
Electronic data capture EDC is the computerized collection and management of clinical trial data from patients and subjects. They are also usually much safer due to the myriad of ways electronic data is stored and secured. Annonce STRUCINSPECT DCE Data Capture Expert training for remote data capturing.
Electronic Data Capture EDC is the medical abbreviation is admittedly a fairly generic sounding term but in the clinical trials field it actually means something fairly specific. Electronic Data Capture thats tailor-made for MedTech studies. Data is typically first recorded on paper and is then transcribed into the system and saved in an electronic case report form eCRF.
Electronic data capture solutions are digital solutions that help to streamline and simplify the collection and usage of data. Optical character recognition can also be a component of data. Annonce Electronic Data Capture for clinical investigations and post market clinical follow-up.
Save time empower your teams and effectively upgrade your processes with access to this practical Electronic data capture Toolkit and guide. Save time empower your teams and effectively upgrade your processes with access to this practical Electronic data capture Toolkit and guide. Data capture or electronic data capture is the process of extracting information from a document and converting it into data readable by a computer.
Provides you with the ability to track manage and monitor disclosures on an ongoing basis. The process reduces data errors to provide researchers with improved data. Using systems to collect clinical trial data in electronic form as opposed to paper form.
Annonce Electronic Data Capture for clinical investigations and post market clinical follow-up. It validates the captured data and makes it readily available for analysis and reporting. Contact us today for a consultation.
Level up your career in data capturing structural assessments and lifecycle analysis. Edcsystem clinicalresearch electronicdatacapture clinicalgyanEDC- Electronic Data Capture Systems as mainstream in clinical researchThis video explains. The age gate tool is available on HALO Pro.
Contact us today for a consultation. Level up your career in data capturing structural assessments and lifecycle analysis. Electronic Data Capture thats tailor-made for MedTech studies.

Top Electronic Data Capture Software Posts By Dacima Data Capture Electronic Data Capture Data

Clinical Data Management Software Offline Electronic Data Capture Electronic Data Capture Software For Cli Electronic Data Capture Data Capture Make It Simple

Pin On British Biomedicine Institute Offers Nanodegree

Electronic Data Capture Here To Stay Infographic Electronic Data Capture Clinical Research Electronic Data Systems

Openclinica Clinical Data Management Epro And Ecrf

Centralised Monitoring Electronic Data Capture Data Capture Clinic

Clinical Program Leader Docs Global Continental Europe Stockholm Sweden In This Role You Will Lead And Manage The Cross Job Board Job Science Degree
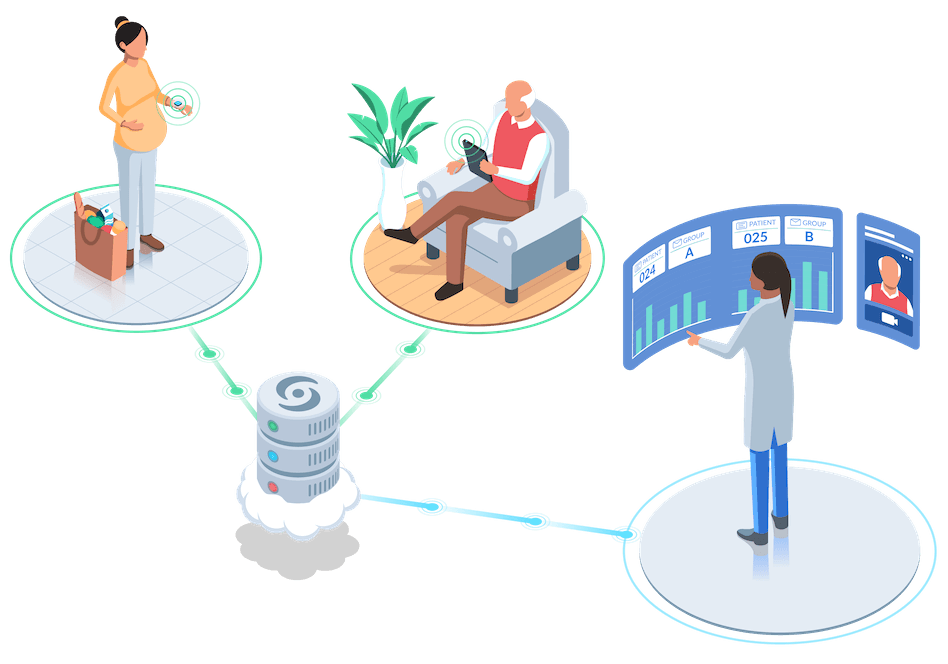
Electronic Data Capture Edc Econsent Ecrf Epro Clinical Research Castor

About Mainedc Edc And Iwrs System

Clinion Clinical Trials Solutions Help To Provide A Global Approach To Your Research Work By Creating A Wo Electronic Data Capture Data Capture Clinical Trials

What Is Electronic Data Capture Software Development Web Development Web Development Design

Ford Ford Etis Offline 2020 02 Ford Ecat 2016 01 Vmware Html Page
Https Www Ema Europa Eu En Documents Regulatory Procedural Guideline Draft Guideline Computerised Systems Electronic Data Clinical Trials En Pdf

What Is Ecoa And How Does It Improve Clinical Trial Data Quality

Electronic Data Capture Edc Econsent Ecrf Epro Clinical Research Castor

Fixing Concerns By Using Data Capture Services Data Capture Electronic Data Capture Data



Post a Comment for "How Does Electronic Data Capture Work"